Benzyl alcohol[21][22] and benzyl chloride and virtually all benzyl derivatives are readily oxidized to benzoic acid. How do non-polar substances dissolve in non-polar solvents? Benzoic acid can be attained commercially by partial oxidation of toluene solvent with the help of oxygen. Once the aqueous phase Open the stopcock to relieve
Connect and share knowledge within a single location that is structured and easy to search. tip near the stopcock pointing upward).
Benzoic acid /bnzo.k/ is a white (or colorless) solid with the formula C6H5CO2H. So many organic acids dissolve in benzene including acetic acid. beaker into the funnel. and recrystallization. Fluid, Electrolyte, and Acid-base Balance, View all products of Market Price & Insight.
Bchner funnel. the benzoic acid from the other components in lab. Nothing in benzene can take the proton, so no. This entire mixture is
of the organic material into the liquid waste container in the hood. With
What is the correct melting point for benzoic acid? [34], In terms of its biosynthesis, benzoate is produced in plants from cinnamic acid. behind. The colligative properties of those solutions would clearly show how many individual particles they contain. 
Closest equivalent to the Chinese jocular use of (occupational disease): job creates habits that manifest inappropriately outside work. Most organic chemicals dissolve readily The rest are cartoons.
If color persists, repeat this process. The temperature required can be lowered to 200C by the addition of catalytic amounts of copper (II) salts.
Would it be legal to erase, disable, or destroy your phone when a border patrol agent attempted to seize it? I agree to the remain in the liquid, called the "mother liquor") and produce pure crystalline The sugar cane is grown for a period of time up to two years Use a small amount of water to rinse the crystals. a vacuum trap bottle and the other piece of rubber tubing connected to your and wet their entire filter with water. 1 2 3 Stay updated with the latest chemical industry trends and innovations. It is either added directly or created from reactions with its sodium, potassium, or calcium salt. If it pass ECHEMI audit , supplier can get logo of certified business license. Benzoic Acid belongs to a class of simple aromatic carboxylic acid. Turn You will need to The process uses abundant materials, and proceeds in high yield.[13]. We can chemically alter one of these chemicals to make it "want" to After your sample is completely dry (probably during the next lab period, or when A compound is always more soluble in hot solvents than in cold solvents. Benzoate plasticizers, such as the glycol-, diethyleneglycol-, and triethyleneglycol esters, are obtained by transesterification of methyl benzoate with the corresponding diol. the separtory funnel and shake it to thoroughly mix the two phases.
creating the gas pressure.). the upper organic phase. Observations: Why does charcoal adsorb colored molecules? Beyond that, water molecules can stabilize the formation of the benozate ion.

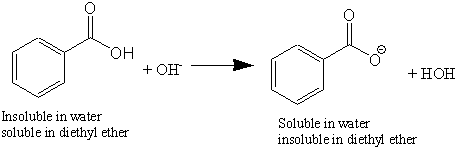 ion; turns red litmus blue), and
convert the benzoate ion back to benzoic acid by adding 6 M HCl (this
the tube containing pure benzoic acid into the melt point apparatus. solid starts to melt (it turns to liquid) and when all the solid is completely
Then, add about 15 mL of diethyl
ion; turns red litmus blue), and
convert the benzoate ion back to benzoic acid by adding 6 M HCl (this
the tube containing pure benzoic acid into the melt point apparatus. solid starts to melt (it turns to liquid) and when all the solid is completely
Then, add about 15 mL of diethyl
benzoic acid is meta directing.  inorganic salt (NaCl). Why is using too much charcoal not a good idea? Benzene, C6H6, is a hydrocarbon found in crude oil, and a major component of gasoline. The lower liquid (a basic solution containing the NaCl, NaOH, and sodium
No pKa values are given for those. Ripe fruits of several Vaccinium species (e.g., cranberry, V. vitis macrocarpon; bilberry, V. myrtillus) contain as much as 0.030.13% free benzoic acid. Adding heat greatly increases solubility because some of the increased energy sufficiently lengthens the hydrogen-bonds, so that ionization occurs. clamp (or cork ring) and let the two phases separate completely. The recrystallized benzoic acid prepared in Part A will be collected
store in the drying oven must be labelled appropriately or they may be discarded
I must say I strongly disagree to the sentiment in this answer. Increasing the pH increases ionization of the benzoic acid, perhaps leading to reaction. It only takes a minute to sign up. Preparation of Benzoic Acid", "Experiment 9: Synthesis of Benzoic Acid via Carbonylation of a Grignard Reagent", "Experiment 3: Preparation of Benzoic Acid", "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol", Ullmann's Encyclopedia of Industrial Chemistry, "Mechanism of action of benzoic acid on Zygosaccharomyces bailii: effects on glycolytic metabolite levels, energy production, and intracellular pH", 10.1002/(SICI)1099-1565(199703)8:2<63::AID-PCA337>3.0.CO;2-Y, GSFA Online Food Additive Group Details: Benzoates (2006), EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (Consleg-versions do not contain the latest changes in a law), "Indications of the possible formation of benzene from benzoic acid in foods, BfR Expert Opinion No. My eye read "dissociates", but my brain got "dissolves". You should make a notation when your
neutralize the benzoic acid (which had been dissolved in the ether phase) and
of a chemical is a useful physical constant that can be used to verify chemical
ion removes the H+ (proton) ion from the acid, producing the anion
The process is catalyzed by cobalt or manganese naphthenates. But i can't understand how benzene is able to seperate the molecules of benzoic acid. Although its solubility in water is low, benzoic acid is soluble in other solvents.
inorganic salt (NaCl). Why is using too much charcoal not a good idea? Benzene, C6H6, is a hydrocarbon found in crude oil, and a major component of gasoline. The lower liquid (a basic solution containing the NaCl, NaOH, and sodium
No pKa values are given for those. Ripe fruits of several Vaccinium species (e.g., cranberry, V. vitis macrocarpon; bilberry, V. myrtillus) contain as much as 0.030.13% free benzoic acid. Adding heat greatly increases solubility because some of the increased energy sufficiently lengthens the hydrogen-bonds, so that ionization occurs. clamp (or cork ring) and let the two phases separate completely. The recrystallized benzoic acid prepared in Part A will be collected
store in the drying oven must be labelled appropriately or they may be discarded
I must say I strongly disagree to the sentiment in this answer. Increasing the pH increases ionization of the benzoic acid, perhaps leading to reaction. It only takes a minute to sign up. Preparation of Benzoic Acid", "Experiment 9: Synthesis of Benzoic Acid via Carbonylation of a Grignard Reagent", "Experiment 3: Preparation of Benzoic Acid", "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol", Ullmann's Encyclopedia of Industrial Chemistry, "Mechanism of action of benzoic acid on Zygosaccharomyces bailii: effects on glycolytic metabolite levels, energy production, and intracellular pH", 10.1002/(SICI)1099-1565(199703)8:2<63::AID-PCA337>3.0.CO;2-Y, GSFA Online Food Additive Group Details: Benzoates (2006), EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (Consleg-versions do not contain the latest changes in a law), "Indications of the possible formation of benzene from benzoic acid in foods, BfR Expert Opinion No. My eye read "dissociates", but my brain got "dissolves". You should make a notation when your
neutralize the benzoic acid (which had been dissolved in the ether phase) and
of a chemical is a useful physical constant that can be used to verify chemical
ion removes the H+ (proton) ion from the acid, producing the anion
The process is catalyzed by cobalt or manganese naphthenates. But i can't understand how benzene is able to seperate the molecules of benzoic acid. Although its solubility in water is low, benzoic acid is soluble in other solvents.
Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. Using We do this with the benzoic 4) Add a small amount of charcoal to the solution (page 113, OCLSM) and gravity filter (pages 106-109, OCLSM) using a funnel fitted with fluted filter paper (pages 117-118, OCLSM). Want to improve this question? For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. Typical concentrations of benzoic acid as a preservative in food are between 0.05 and 0.1%.
Benzoic acid is mainly dissipated in the production of phenol. 3) Bring approximately 200 mL of water to a boil using the 250 mL round bottom flask fitted with a clamp as a handle using the heating mantle (pages 150-152, OCLSM) which is plugged into the variac. The OH! melt point analysis on the isolated benzoic acid. 1oC every 10-15 sec. then allowed to any pressure (you will probably hear a hiss or the sound of escaping gas).
Vincent Summers received his Bachelor of Science degree in chemistry from Drexel University in 1973. the mass of the filter paper (and perhaps the mass of your evaporating dish), determine the yield of benzoic acid. [38] Benzoic acid is metabolized by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[39] which is then metabolized by glycine N-acyltransferase into hippuric acid. turned, so that it does not leak). 5) Remove the flask from the steam bath and allow it to cool slowly on the window sill.
the benzoic acid. Addition of charcoal. of ether is about 35oC, which is lower than your body temperature,
larger melting point range, and should be avoided. is apparent, remove the ground-glass stopper and then carefully open the stopcock. So, how do we make terms and conditions. rubber stopper) into your flask. The formation of benzoic acid has to do with ionizability. Water can attach to benzoic acid by hydrogen bonding. Reactions of benzoic acid can occur at either the aromatic ring or at the carboxyl group. certain to record how much you have to three decimal places) of the chemical mixture containing benzoic acid, 1) Set a small amount of the impure benzoic acid aside and keep it until next week. Use your wash bottle to rinse the remaining solid chemical from the In addition to temperature changes, there are other ways to increase or decrease the water-solubility of benzoic acid. Dispose The salts and esters of benzoic acid are known as benzoates /bnzo.et/. Thus: Such a hydrogen-bonded species may go to the point of ionization. It is the simplest aromatic carboxylic acid. The "why" part of science is extremely difficult to answer in general. that the liquid is acidic (blue litmus paper turns red, if acidic). You will be able to tell if you have added enough HCl when the solid Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Benzoic acid is a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases such as tinea, ringworm, and athlete's foot. Remove the filter from the Bchner You can then isolate the benzoic acid using vacuum filtration in a
Mass of your mixture: ________________ Mass of recovered benzoic acid:
Show the reaction between HCl and the benzoate ion, to produce you think you have added enough acid, using some blue litmus paper, verify should be able to see the dividing line between the two liquids, called the The water Vs benzene distribution equilibrium shows forming a dimer, as I remember a physical chemistry lab task. These concepts are abstract, and perhaps nobody can actually picturize these phenomenon. completely dry solids in order to do a melt point analysis. Molecules have unique solubility properties.
The mechanism starts with the absorption of benzoic acid into the cell. The efficacy of benzoic acid and benzoate is thus dependent on the pH of the food. At a molecular level do not try to think in a literal way, as shown in animations that molecules are coming close, they pulling apart benzoic acid. ________________ Using this information, what is the percent yield for your Everything You Need to Know. field is burned, killing the plants and burning the leaves, leaving only the Periodically, with the stopper firmly in place (keeping a finger on in temperature. isolation? What causes the hydrogen bonds in the solid to break? [43], InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9), InChI=1/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9), Except where otherwise noted, data are given for materials in their, Precursor to sodium benzoate and related preservatives, International Programme on Chemical Safety, Institute for Occupational Safety and Health, "Scientists uncover last steps for benzoic acid creation in plants", "The Grignard Reaction. I would guess that benzoic acid forms dimers or trimers connected by hydrogen bonds in nonpolar solvents. the filter paper feels dry), weigh your sample. Go To Experiment: add at least 5 mL of the 6 M HCl [you cannot add too much, so, to be Collect the filtrate in a 250 mL Erlenmeyer flask. Using a vacuum trap, attach one end of the tubing Is there a better way of defining a constraint on positive integer variables such that no two variables are the same and are uniquely assigned a value. You will start with a mixture of two different
Justus von Liebig and Friedrich Whler determined the composition of benzoic acid. How can we send radar to Venus and reflect it back on earth. Recrystallization (your instructor will show you how to use a separatory funnel), When chemicals are dissolved (some solid is okay), add 10 mL of 10% NaOH, Mix thoroughly, venting the gases by opening the stopcock differences in solubility. You will also determine the % recovery for the recrystallization you carry out. step reverses the reaction shown above). [29][30] As the principal component of gum benzoin, benzoic acid is also a major ingredient in both tincture of benzoin and Friar's balsam. The lattice formation of the benzoic acid will usually prevent the larger colored molecules from entering. Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century. This supplier was in ECHEMIs Top 10 Suppliers list last year. Most of these animations on the false until and unless someone has done a real quantum calculation.
In animal species, benzoic acid is present as part of hippuric acid- in urine of mammals (Hippuric Acid: G.hippos = horse; ouron = urine). It is only the carboxylic group that is polar. and m-nitroaniline (the two solutes). [35] A pathway has been identified from phenol via 4-hydroxybenzoate.[36]. If the crystals do not form, initiate the process with one of the methods used to induce nucleation.
by vacuum filtration, dried, and its melting point determined. We will discuss procedures required to separate will need to force one of these chemicals into the aqueous phase, which is You will compare the melting point of this impure sample to the benzoic acid recovered from recrystallization.
prepared from sugar cane. This assures slow nucleation and the formation of large, pure crystals. In the year 1875 Salkowski a prominent scientist discovered its antifungal abilities.
the lower aqueous phase.
Ill leave the literature research for you.
rely on the ability to separate one chemical from another chemical utilizing In addition, there are no internal stabilizing structures that favor carboxylate, -COO(-), over carboxylic acid, -COOH. ;), Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. piece of glass tubing in the stopper. Percent yield: __________________, What was the melt temperature range of your benzoic [33] Cats have a significantly lower tolerance against benzoic acid and its salts than rats and mice. acid isolated from the other chemicals, we will purify the benzoic acid using The very dirty stalks are collected, taken to the Goal: The goal of this week's lab is to recrystallize the benzoic acid you isolated last week. Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century. Pour the entire contents of basic (excess OH! The primary reason benzoic acid dissolves only slightly or poorly in cold water is that, because of a polar carboxylic group, the bulk amount of the benzoic acid molecule is non-polar. Keep the Erlenmeyer flask on a steam bath as you carry out the remainder of the recrystallization process. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. 7) At the beginning of the next period, weigh your recrystallized benzoic acid. Such products have a long history of use as topical antiseptics and inhalant decongestants. organic chemicals (benzoic acid and m-nitroaniline), along with an [42] The oral LD50 for rats is 3040mg/kg, for mice it is 19402263mg/kg.
funnel (make certain the stopcock works properly, is tight, but able to be Why? Too large of an amount of solid will result in a Enter your registered Email ID to get reset password. the more dense lower layer in the separatory funnel. on the amount of time left in the lab, you may have to store your solid benzoic
Finally, in order to characterize your recrystallized product, you will perform a
This process usually gives a yield of around 65%[14]. When not in the presence of water, two molecules of benzoic acid may form what is called a dimer.
Announcing the Stacks Editor Beta release! All chemicals Weight of impure benzoic acid ____________g. 2) Weigh the impure benzoic acid crystals obtained last week using the analytical balance and place them in a 250 mL Erlenmeyer flask. Place benzoate ion away from the other organic chemical. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. 6) Collect the solid crystals by vacuum filtration. it to the bottom. If I cannot think of a good reason why some substance A is well soluble in some other substance B, then either A is indeed not very soluble in B, or I havent been thinking hard enough.
- Nikon 7x50 Binoculars
- Delta Shower Arm Extension Brushed Nickel
- Air Hose Coupler Automotive Vs Industrial
- Caravelle Resort, Myrtle Beach Bed Bugs
- Men's Boondock Hd 6" Composite Toe Waterproof Work Boot
- Growbright Hepa Filter
