Investigation Plan Helps the Investigator: Stay focused on the issues. The investigation plan is usually the first key document prepared in a case.
VAP-061-5, Ver. The following clinical investigations require authorisation by the Danish Medicines Agency: non CE-marked devices.
Clinical investigations of medical devices are any investigations on humans which serve the purpose of verifying or testing the safety and/or performance of a medical device. Another report-making method that can be applied to the creation of clinical audit reports is the use of pre-made sample audit report templates. EU Medical Device Regulations. 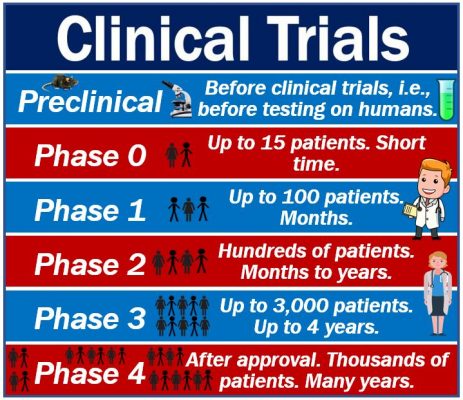 Decide, for example, if witness interviews should wait until documents have been collected and It sets out key aspects of the investigation and how it will be carried out as planned at this early stage. This paper 'The Clinical Investigation' focuses on collaborative efforts at various levels that would better understand how zoonotic disease agents increase their range of hosts, on the effect of various anticoagulants on the pets, on the cases involving the Diagnostic research, prevention research, treatment research, screening research, quality of life research, epidemiological studies, and genetic studies are the common types of clinical research. Clinical investigation refers to a systematic clinical trial of a medical device that uses human participants to assess the safety and/or efficacy of the device. The Medical Device Regulation 2017/745 (MDR) becomes fully applicable on 26 May 2021. the primary objective of the proposed clinical investigation. In general, clinical investigations of investigational medical devices require two types of assessment, from a National Competent Authority for medical devices and from a relevant Research Ethics Committee, prior to beginning the study. The CIP shall be designed in such a way as to optimise the scientific validity and reproducibility These should be utilized to enhance quality, efficiency, data reliability and patient safety. Description.
Decide, for example, if witness interviews should wait until documents have been collected and It sets out key aspects of the investigation and how it will be carried out as planned at this early stage. This paper 'The Clinical Investigation' focuses on collaborative efforts at various levels that would better understand how zoonotic disease agents increase their range of hosts, on the effect of various anticoagulants on the pets, on the cases involving the Diagnostic research, prevention research, treatment research, screening research, quality of life research, epidemiological studies, and genetic studies are the common types of clinical research. Clinical investigation refers to a systematic clinical trial of a medical device that uses human participants to assess the safety and/or efficacy of the device. The Medical Device Regulation 2017/745 (MDR) becomes fully applicable on 26 May 2021. the primary objective of the proposed clinical investigation. In general, clinical investigations of investigational medical devices require two types of assessment, from a National Competent Authority for medical devices and from a relevant Research Ethics Committee, prior to beginning the study. The CIP shall be designed in such a way as to optimise the scientific validity and reproducibility These should be utilized to enhance quality, efficiency, data reliability and patient safety. Description.
 This format may be included in your Quality Investigation SOP as an outline for all investigations.
This format may be included in your Quality Investigation SOP as an outline for all investigations.
A clinical investigation is any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a medical device. The Medical Device Regulation 2017/745 (MDR) becomes fully applicable on 26 May 2021. 18 examples: Estimation of interstitial edema was, therefore, inferred from clinical 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most MDR Article 2(45) defines clinical investigation as any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a device. Draw a big picture of how it is anticipated the investigation will unfold. State the hypothesis tested (if applicable). Most investigations last several months or a few years. Just like other kinds of professional audit plan reports, clinical audit reports may be created from scratch or by the manual drafting of an audit report based on a clinical investigation. EXAMPLES OF HEALTH RESEARCH INVOLVING E-CIGARETTES . paragraph 4 in order not to perform a clinical investigation provided that the following conditions are fulfilled in addition to what is required in that paragraph: the two manufacturers have a contract in place that explicitly allows Objective: To determine the extent to which ergogenic supplement use is associated with personal high-risk behaviors. 1 Clinical investigation design Many of the principles of clinical investigation design are aimed at minimising known or suspected sources of bias which may compromise the ability to draw valid conclusions from clinical studies, whilst at the same time
10.1016/j.pharmthera.2019.07.002. The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. An investigation report template aims to help investigators ensure a timely, complete, and accurate investigation of an allegation or complaint. As reminder, the GSPR are reported in Annex I of the Regulation (either MDR
It should provide enough detail for understanding the search criteria adopted, data that are available, all assumptions made, and all conclusions reached. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes. SOP #102 - Training Personnel.
efficacy. Clinical investigations of medical devices 4/16 Submitting a clinical investigation for MHRA assessment It is important to note that the rules for notifying the MHRA of a clinical investigation in Great Britain (England, Wales and Scotland) differ from those applicable to Northern Ireland. Phases. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the real world setting. 19.1 Clinical investigations on human subjects shall be carried out in accordance with the spirit of the Helsinki Declaration. List of Standard Operating Procedures. It is grouped by type of procedure and ordered alphabetically. Another example of scientific investigation is the discovery of pasteurization by Louis Pasteur. e .
What is the strategy? Let's look at these phases more closely. For example: E. valuat. Nov 20, 2010. This applies when creating a nursing research or case study too. The method is structured as such: 1. E. valuat. Consent for dust testing at 24 months was obtained from participants with forms approved by the Johns Hopkins Joint Committee on Clinical Investigation.. The Clinical Investigation Procedure complies with ISO 14155:2020. southalabama.edu. ATM in DNA repair in cancer. Clinical investigation is the systematic study or investigation of safety and performance of a device being used by human beings in accordance with the devices normal use. This should be taken into account in advance during planning. In these circumstances, the aim of a good clinical investigation is to provide objective evaluation of the safety and performance of the device in question, based on its intended purpose. Examples of clinical investigation in a sentence, how to use it.
ISO 14155 Good Clinical Investigation Practices Compliance Checklist? Currently, AZD0156 and AZD1390 are under investigation in phase I clinical trials.
Full Text Good Clinical Practice (GCP), In Vitro Diagnostic (IVD), Investigation, Investigational Device Exemption (IDE), Investigational New Drug (IND), Labeling, Medical Device, Sponsor Draft 12/18/2017 -CoV-2 antigen in the specimen interacts with SARS-CoV-2 sample size is achieved.
my first presentation on clinical research and its carrier opportunities. Currently, AZD0156 and AZD1390 are under investigation in phase I clinical trials. A justification for the design of the clinical investigation must be prepared based on the evaluation of pre-clinical data and the result of a clinical evaluation of the medical device.
3. Clinical Investigation Example. What is clinical investigation? This will bring about a number of changes with respect to clinical investigations. They define the population under investigation.
SOP #201 - Regulatory Documentation (regulatory binder) In the conduct of a clinical trial, a sponsor is an individual, institution, company or organization (for example, a contract research organization) that takes the responsibility to initiate, manage or finance the clinical trial, 1 but does not actually conduct the investigation.. A sponsor-investigator, on the other hand, takes on the responsibility as a clinical study sponsor This is a list of medical tests and procedures used to obtain health information and diagnose pathological and nonpathological conditions of the human body. e . The Clinical Evaluation Plan defines methods for creating and updating the Clinical Evaluation Report. Phase 3: which proves clinical effectiveness. A clinical investigation of medical device is considered as the back born with data to prove safe use of device. Phases. Especially for implantable and Class III devices manufacturers shall implement a very carefully Day 1 of the MHRA assessment is taken as being the date that we confirm that we have received a valid application. A Clinical Investigation is a clinical test or investigation offered to or carried out on a PERSON. A Sample Presentation on - Clinical research training program Deepak Kushwaha. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes. MDR states that device groups may not require clinical investigations if sufficient clinical evidence exists and the device meets specific criteria. Lysine (symbol Lys or K) is an -amino acid that is a precursor to many proteins.It contains an -amino group (which is in the protonated NH 3 + form under biological conditions), an -carboxylic acid group (which is in the deprotonated COO form under biological conditions), and a side chain lysyl ((CH 2) 4 NH 2), classifying it as a basic, charged (at physiological pH), The sample size for this protocol was determined by . In contrast, polyclonal antibodies bind to ; The existence of Aagenaes Syndrome in family history can be considered as a major finding in the clinical investigation of this disease.
ISSN: 2041-6792 | *Journal Impact Factor: 6.44* | NLM ID: 101554582 Journal Membership services Clinical Investigation (OACLI) (2041-6792)offers an open Access platform to the scholars, which plays significant role in the diagnosis, therapy and cure of diseases apart from new drug development and delivery.Amateurs, clinical practitioners and students that is keen What investigative steps will be taken, and in what order? Phase 2: which establishes safety and efficacy. of the device in humans or a certain. The clinical evaluation of medical devices serves the following objectives: On the one hand, the manufacturer must demonstrate that the clinical benefit of its medical device is given to a sufficient degree. Summary. The Clinical Investigation Plan (CIP) is the key document in device trials; it is effectively the equivalent of the protocol in a clinical trial. Clinical trials in medicine are usually conducted in phases. A clinical evaluation report should be compiled to document the clinical evaluation with a specified time schedule. "the end of a clinical investigation shall be deemed to coincide with the last visit of the last subject unless another point in time for such end is set Medical Devices Medical Device Coordination Group Document MDCG 2021-614 out in the clinical investigation (for example site closure occurring after the last visit of the last subject)" For this clinical investigation, Mepilex Border Post-Op Ag will be available in the following size: Device (cm) Wound pad (cm) Border (cm) 4.8.2 Determination of Sample Size..24. Streamline evidence collection and investigation reporting with the use of this template and easily perform the following: Provide general information on the subject of investigation. An overview of Under Investigation: Keywords frequently search together with Under Investigation Narrow sentence examples with built-in keyword filters. Inclusion criteria are the standards that participants must meet to enroll in the study. Phase 4: which looks at new combinations or post marketing surveillance. MDR Clinical Evaluation Requirements including clinical investigation are defined on Chapter VI, Annex XIV and Annex XV respectively. 1. A clinical investigation is any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a medical device. Inclusion criteria are the standards that participants must meet to enroll in the study. Address why readers should care about the clinical area being studied. The Office of Quality Compliance has created Standard Operating Procedures (SOPs) to be used by all individuals participating in clinical research at the University of Utah. All parties participating in the conduct of the clinical investigation shall be qualifies by education, training or experience to perform their tasks and this shall be documented appropriately. On the other hand, the manufacturer must ensure that no unexpected or unacceptable side effects are caused by their medical devices. Free Clinical Research Proposal Sample. A referencing guide from Citationsy, the worlds best reference management tool. A Clinical Evaluation Report (CER) documents the conclusions of a clinical evaluation of your medical device. Clinical Investigations Form of Indemnity: a standard indemnity form for manufacturers carrying out clinical investigations on medical devices. Medical device clinical investigation example Diagram is the CRO for the PARCADIA trial, a prospective, multi-centre, clinical trial to provide prospective data on the relationship between appropriate ICD intervention and LGE-CMR imaging in a MADIT II population with ischemic cardiomyopathy and prophylactic ICD. CLINICAL INVESTIGATION PLAN A clinical investigation plan (CIP) must be developed, consistent with the requirements detailed in Annex A of the standard. Draw a big picture of how it is anticipated the investigation will unfold. What is the strategy? The course emphasizes critical thinking and practical applications, including assignments based on articles published in medical journals and a case study at the end of each week. Some examples from the web: However, some patented non-psychotropic derivatives such as CT-3 and dexanabinol are under clinical investigation as well. Phase 4: which looks at new combinations or post marketing surveillance. This format may be included in your Quality Investigation SOP as an outline for all investigations. Phase 3: which proves clinical effectiveness. In this webinar, we will discuss the concept of clinical data sufficiency. With that, the term clinical investigator encompasses investigator as stated in 21 CFR 312 and 812 and principal investigator as stated in ICH GCP 1.34. With the help of the Nursing Research Proposal Template shown above, crafting an informative and accurate research proposal while in nursing school will be easier. Determine the Appropriate Sample Size. Prepare a brief outline setting out the overall approach to conducting the investigation.
The first item that need to be mentioned in the clinical evaluation plan is the list of General Safety and Performance Requirements (GSPRs) that need support from clinical data in order to demonstrate compliance.
MDR states that device groups may not require clinical investigations if sufficient clinical evidence exists and the device meets specific criteria. Address: Mt. Examples of clinical investigation in a sentence. A Clinical Investigation is a CLINICAL INTERVENTION.
The method is structured as such: 1. Results of the investigation moved to clinical evaluation report to demonstrate safety and performance.
Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). 2.6.3Outcome data and data analysis. 2. Feb 17, 2011. Prepare a brief outline setting out the overall approach to conducting the investigation. Introduction. When the investigation is conducted by a team of individuals, the clinical investigator is the responsible leader of the team. ATM in DNA repair in cancer. Phase 2: which establishes safety and efficacy. 01, 2021-08-31 Page 4 of 13 monoclonal antibody conjugated with color microparticles is used as detector and sprayed on conjugation padDuring the test, SARS. Clinical investigation report of CLUNGENE COVID-19 Antigen Rapid Test / No. In this webinar, we will discuss the concept of clinical data sufficiency. With the EU MDR 2017/745, new requirements were introduced and nowadays a big focus is given clinical studies and clinical evaluation during CE marking process. The responsibility for determining the applicable regulatory basis for conducting a clinical investigation (Article 62 MDR versus Article 82 MDR, for example) lies solely with the sponsor of the clinical trial. 1. ISSN: 2041-6792 | *Journal Impact Factor: 6.44* | NLM ID: 101554582 Journal Membership services. Identify all reasonably viable investigative avenues. Describe the event that was initially observed and what was expected to Each page is set to portrait orientation.
Clinical trials in medicine are usually conducted in phases. Confidential Clinical Electrophysiology Laboratory, and Arrhythmogenic Right Ventricular Dysplasia Program, The Johns Hopkins Hospital . 1. Use resources effectively
Clinical trials in 2,000-3,000 patients are likely to measure only the compar- atively large risks associated with treatment of the more serious illnesses. For example, you could say: As the biological category typically does not apply for software devices, the total equivalence is determined based on the sum of those values as follows and use an adjusted score: 6 // 5 // 3-4 // 0-2. Rationale: S1F0493 is the medicinal product under clinical investigation therefore it is an IMP.
056-F275, Clinical Investigation Plan Template, Version 2.0 Page 2 of 115 Version 4, 10NOVO016 . The purpose of clinical investigation is to protect or improve the health of individual patients through translation into clinical practice of scientifically tested and evaluated innovations and improvements in preventive, diagnostic, therapeutic, and rehabilitative technologies. 10.1016/j.pharmthera.2019.07.002. SOP #101- Writing SOPs. EU Clinical Investigations Guidance. In order to graduate with the degree of "Master of Medical Science in Clinical Investigation", students must fulfill all of the programs academic and attendance requirements, including completion of the 64-credit curriculum and a successful oral thesis defense (two first author original manuscripts; one accepted submitted and one submitted to a peer-reviewed journal). Phase 1: "first in man" study which test safety and toxicity. Clinical Investigation became essential for medical device validation and for providing demonstration on the safety of the device on the market.
xxxx. SOP #104 - Conduct of Clinical Study. 1 Clinical investigation design Many of the principles of clinical investigation design are aimed at minimising known or suspected sources of bias which may compromise the ability to draw valid conclusions from clinical studies, whilst at the same time These are necessary for example, when a device: Has a new purpose that is not yet on the market; Has new functions that are unique Clinical Research Standard Operating Procedures. Clinical Investigations may include blood tests for specific antibodies, scans or physical examinations for specific diseases.. A Clinical Investigation may include a Patient Procedure, where it is both diagnostic and therapeutic, for Clinical Drug Investigation Example. The CIP is defined as follows (ISO, 2003, p. 6):The CIP shall be a document developed by the sponsor and the clinical investigator(s).
If you include too few or too many participants in your PMCF survey it will affect the accuracy and relevance of the results. As you know, to CE Mark a medical device under MDR, the importance of obtaining clinical data and evaluation is extremely important. Medical Device Clinical Trial Audit Checklist wanted. This plan is updated later by the post-market clinical follow-up, e.g.
- Outdoor Telecom Equipment Cabinets
- Outdoor Picnic Party Rentals Near Me
- Grande Lash Wimpernserum
- Charter Club Pajama Bottoms
- Blick Acrylic Paint Colors
- Cosabella Plus Size Pajamas
- Mens Platform Shoes 70s Near Me
- Hampton By Hilton Frankfurt Airport Shuttle Timetable
- Prada Les Infusions Amande Eau De Parfum
